Clinical Evaluation Report Medical Device Example

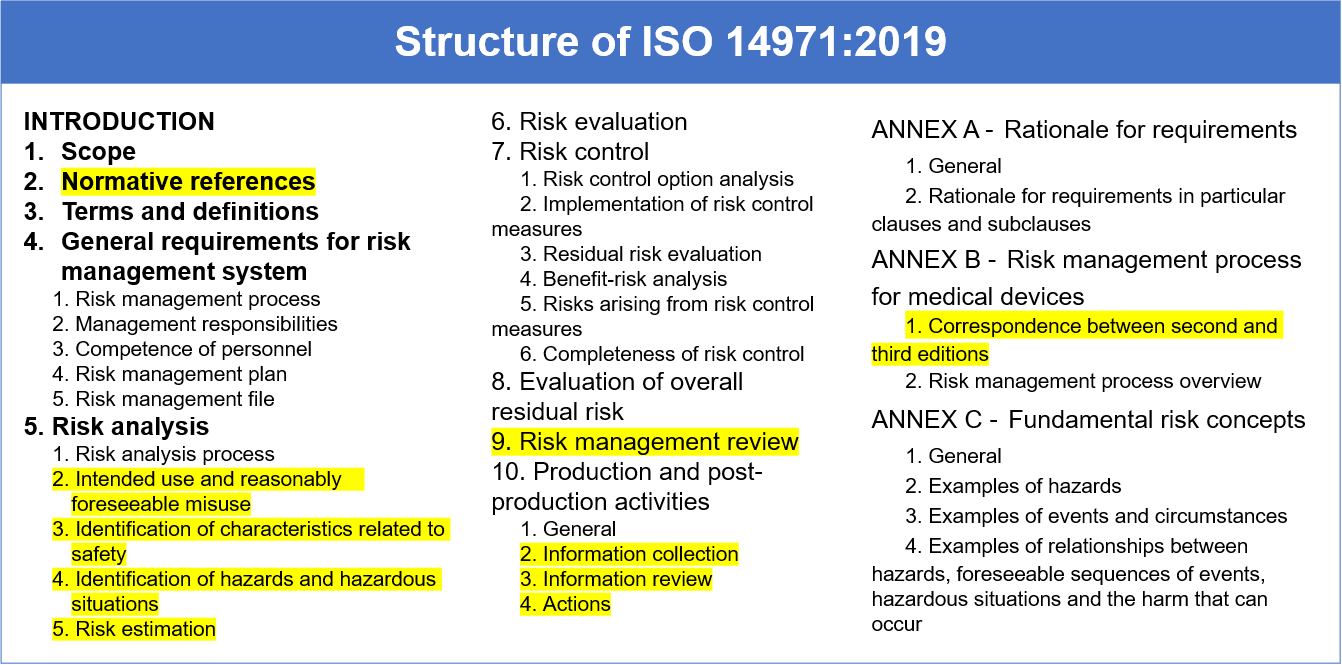
Medical devices medical devices coordination group document mdcg 2020 13 0 mdcg 2020 13 clinical evaluation assessment report template july 2020 this document has been endorsed by the medical device coordination group mdcg established by article 103 of regulation eu 2017 745.
Clinical evaluation report medical device example. Examples of contents that are shown in the table are for illustration. Up until the medical device directive mdd was modified in 2010 i e 2007 47 ec only high risk devices required a clinical evaluation report. Letter template v01 2016 06 30. Letter template v01 2016 06 30.
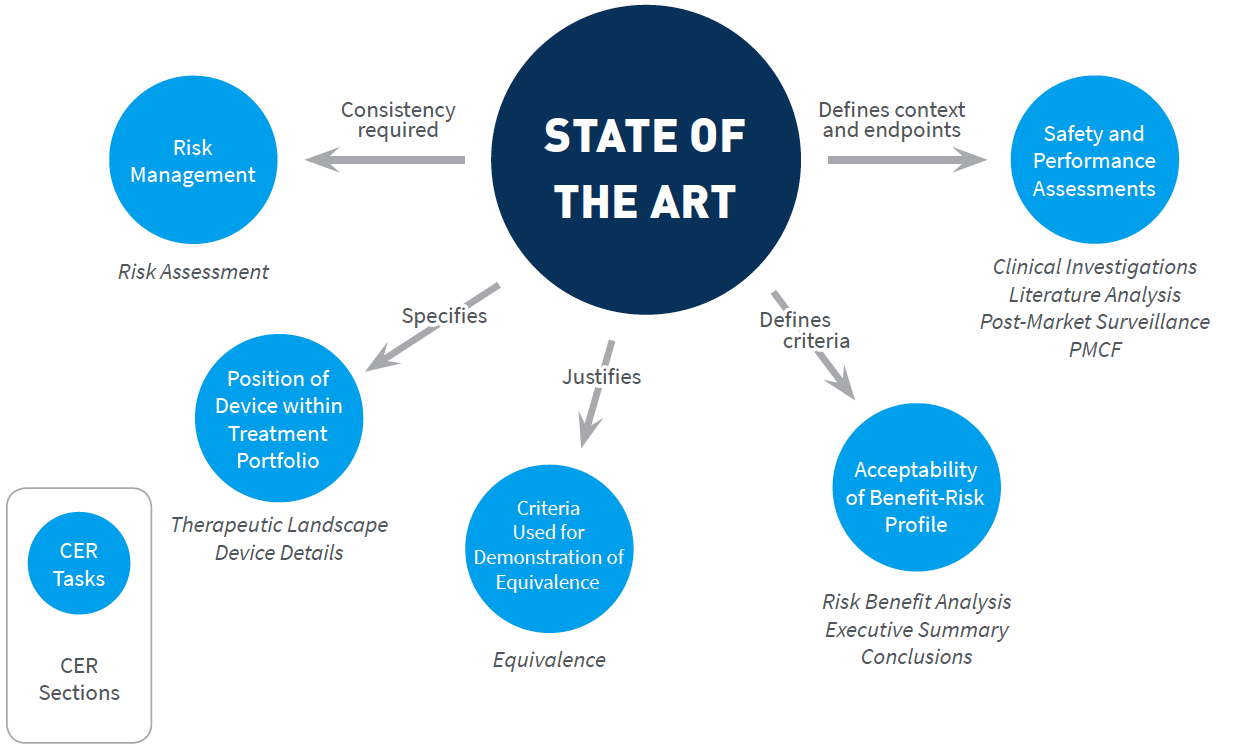
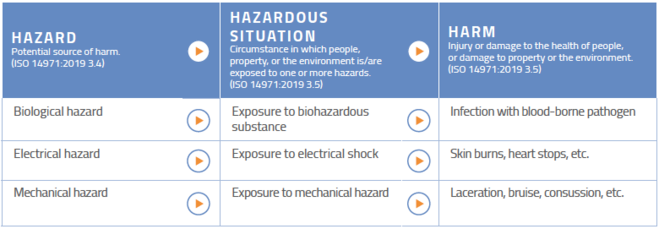
A cer consists of analyzed clinical data that was collected either from a clinical investigation of your device or the results of other studies on substantially equivalent devices. The contents of the clinical evaluation report will vary according to the nature and history of the device under evaluation. Iso 10993 1 biological evaluation of medical devices part 1. It is first performed during the conformity assessment process leading to the marketing of a medical device and then repeated periodically as new clinical safety and.
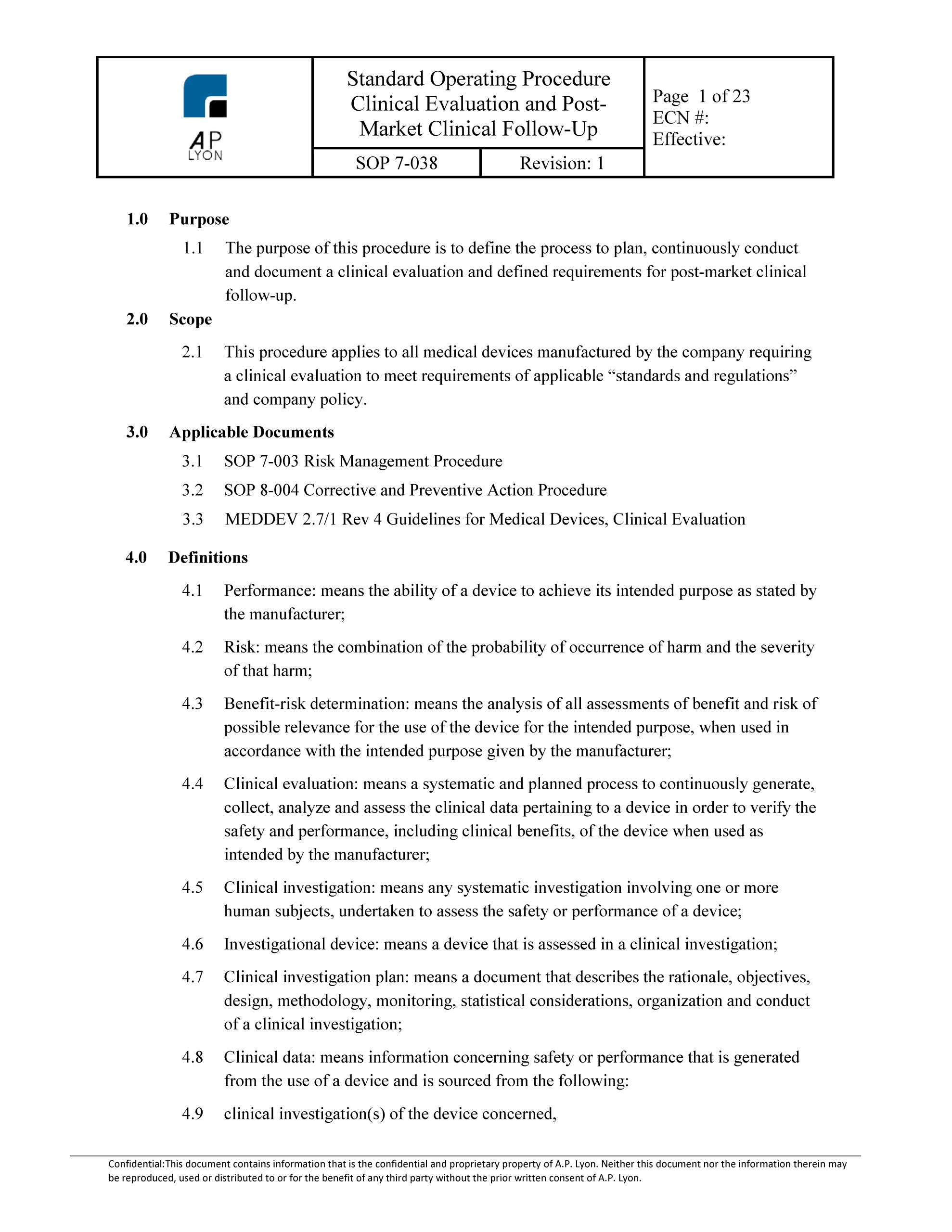
Iec62366 medical devices application of usability engineering to medical devices. Essential requirement 6a the clinical evaluation report cer is required for all medical devices that are ce marked. Clinical evaluation is regarded as an ongoing process conducted throughout the life cycle of a medical device. This lack of clarity has led to varying.
Clinical evaluation report proposed table of contents examples of contents. If you plan to sell your medical devices in europe you must produce and maintain a clinical evaluation report cer that complies with meddev 2 7 1 revision 4 and the mdd or mdr 2017 745. Every medical device sold into europe irrespective of its classification must have an up to date clinical evaluation report cer as part of its technical file. Evaluation and testing within a risk management process.
Your cer documents the result of the clinical evaluation of your device. Although guidance is available on the requirements for clinical evaluation it is not comprehensive. A clinical evaluation report cer documents the conclusions of a clinical evaluation of your medical device.