Ce Mark Medical Device Labeling Requirements

Ce mark medical devices labeling requirements.
Ce mark medical device labeling requirements. This makes the ce marking recognizable. They usually constitute low to medium risk. The full list of these product categories is below. The obl also should review the oem s essential requirements checklist declaration of conformity and ce marking certificates if notified body involvement is necessary for ce marking.
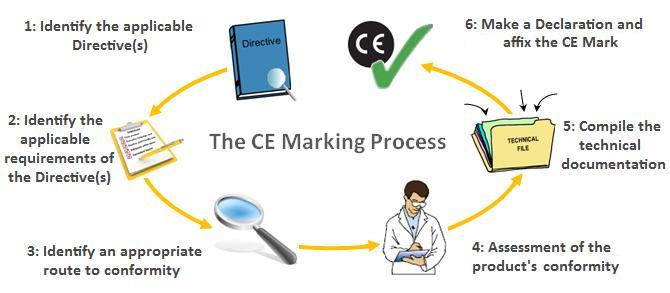
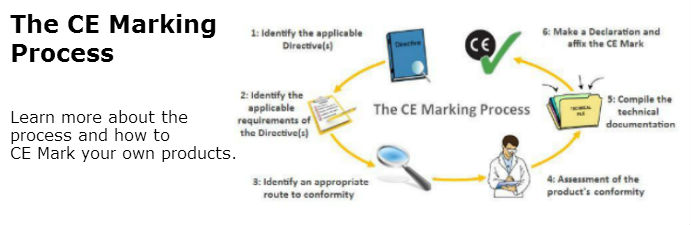
Obtain ce marking and iso 13485 certificates from your notified body. The medical device ce marking process will change when europe s new medical device regulation mdr 2017 745 comes into force in may 2021. It shows that the device is fit for its intended purpose stated and meets. Class iia medical devices.
Ce marking routes of class i medical devices. There are 4 classes class i class iia class iib and class iii. European union ce marking the mark must be accompanied by the id of the notified body when conformity assessment procedures are required. Ce marking is the medical device manufacturer s claim that a product meets the essential requirements of all relevant european medical device directives.
Patients should use them for a short term period any less than 30 days. The directives outline the safety and performance requirements for medical devices in the european union eu. It is not a quality indicator or a certification mark the ce marking is also found on products sold outside the eea that have been manufactured to eea standards. The retail sales packaging.
Ce marking is an administrative marking that indicates conformity with health safety and environmental protection standards for products sold within the european economic area eea. Ce marking applies to products ranging from electrical equipment to toys and from civil explosives to medical devices. There are two major areas of confusion about the translation requirements when ce marking a product for export to the eu states. Prepare a declaration of conformity doc which states that your device complies with the appropriate directive.
Eu foreign language labeling requirements. A ce mark is a logo that is placed on medical devices to show they conform to the requirements in the directives. The ce mark is a legal requirement to place a device on the market in the eu. The device or its sterile package.
Ce mark locations include ce mark. The full list of these. Each medical device is classified into the risks involved.