Ce Mark Medical Device Database Search

Is there any database for list of ce marked medical devices and there current sta such a database does not exist.
Ce mark medical device database search. These products fall under the medical devices legislation and must be ce marked. Class iib medical devices. The medical device ce marking process will change when europe s new medical device regulation mdr 2017 745 comes into force in may 2021. Mdr medical device reporting this database allows you to search the cdrh s database information on medical devices which may have malfunctioned or caused a death or serious injury during the.
The full list of these. The new regulations contain important improvements including a much larger eudamed database than the one that currently exists under the medical devices directives. Instead only the eu national regulators will have access. Examples of medical devices with an ancillary medicinal substance include drug eluting stents bone cement containing an antibiotic catheters coated with heparin or an antibiotic agent and condoms coated.
But this database eudamed will not be publicly accessible. The full list of these product categories is below. Eudamed is the it system developed by the european commission to implement regulation eu 2017 745 on medical devices and regulation eu 2017 746 on in vitro diagnosis medical devices. Here we can include medical devices such as long term corrective contact lenses surgical lasers defibrillators and others.
But it will be introduced by the incoming medical device regulations. Ce marking applies to products ranging from electrical equipment to toys and from civil explosives to medical devices. Ce marking routes of class iia medical devices. Hello i m wondering if there s a centralized publicly available list database of medical devices that have received the ce mark please sign up sign in to read the entire article.
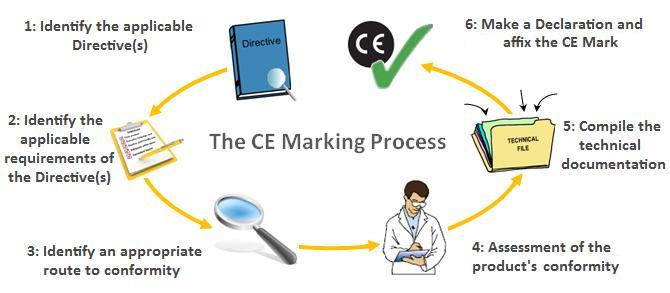
A medical device may contain an ancillary medicinal substance to support the proper functioning of the device. Medical devices notifications mpa in vitro diagnostic medical devices notifications mpivdaoe and address databases with addresses of the persons reporting and of the competent authorities. Prepare a declaration of conformity doc which states that your device complies with the appropriate directive. There are four possible routes to ce mark your product split into two groups given the product s type i e if it s sterile or not.
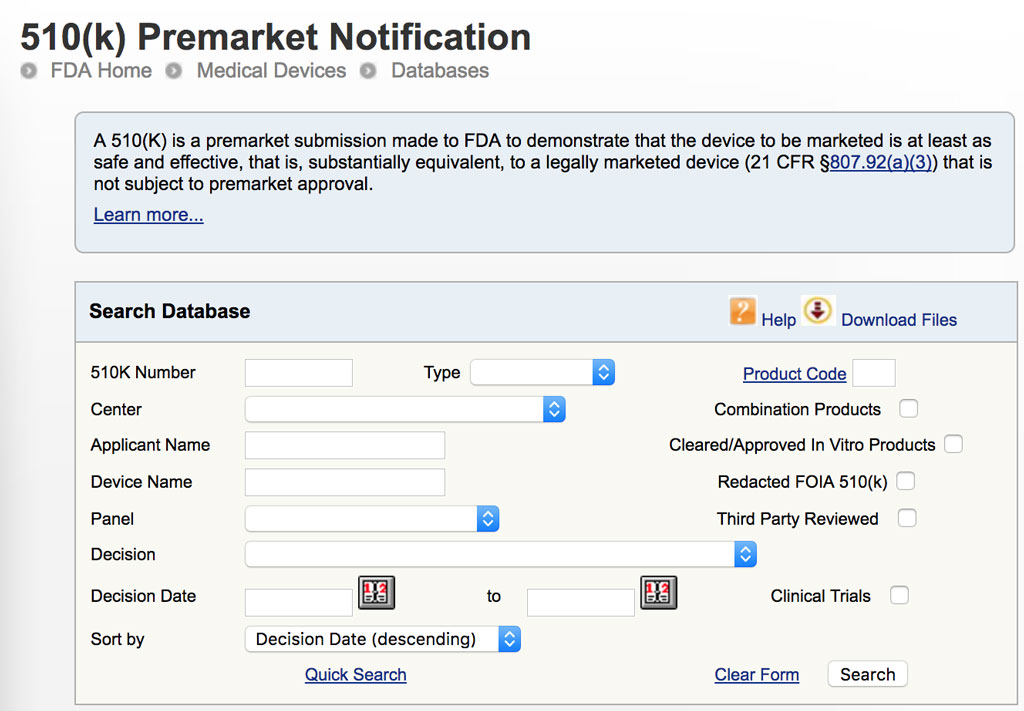
You are welcome to choose any combination of filters. We invite you to search the tris database using the options bellow.